TIEFENBACHER API + INGREDIENTS: Pitavastatine Calcium for novel therapies
TIEFENBACHER API + INGREDIENTS offers Pitavastatine Calcium for novel therapies
We at TIEFENBACHER API + INGREDIENTS help to bridge the gap between drug development and
market access by providing high purity of Pitavastatin Calcium (NK-104 hemicalcium, EU-DMF) to
assure patients have a better access to innovations and novel therapies.
Pitavastatine Calcium (CAS Number 147526-32-7), a relatively new developed cholesterol-lowering
agent (statin), is approved for the treatment of primary hyperlipidemia and mixed dyslipidemia, in
kids > 6 years old and adults. Major clinical benefits from statins appear due to the lowering LDL
levels, directly related to cardiovascular accidents.
Pitavastatine Calcium clinical trials have proved its efficacy in reducing LDL levels in dyslipidemia.
When compared to other statins for its effect to reduce LDL cholesterol, Pitavastatine is about 6-fold
more potent than Atorvastatin, 1.7-fold more potent than Rosuvastatin and 77-fold more potent
than Fluvastatin. Pitavastatine has also demonstrated less potential for the development of myalgia
allowing patients to attain their treatment goal.
Pitavastatine Calcium is immediately available in the highest quality reference for reliable results –
and our whole portfolio includes more than 600+ APIs (Human + Veterinary) to secure the
healthcare industry. For more information around our raw materials with best value seal (Better Access – Better Quality – Better Quality), please contact TIEFENBACHER API + INGREDIENTS: info-api@tiefenbacher.com or visit us on: www.tiefenbacher-api.com

TIEFENBACHER API offers first API based on green chemistry: Enviero Progesterone
TIEFENBACHER API + INGREDIENTS starts to offer the first API based on green chemistry: Enviero Progesterone
We at TIEFENBACHER API + INGREDIENTS are committed to drive green chemistry in the pharmaceutical raw material industry. Initially Enviero Progesterone is an Active Pharmaceutical Ingredient (API) and the first molecule from TIEFENBACHER API + INGREDIENTS’ green-chemistry program – by providing the latest in lower environmental impact products for patients around the world. Suddenly green chemistry is understood to have minimal impacts on the environment and human health, and also to be cost-effective.
In conclusion Enviero Progesterone is produced on a synthesis that reduces waste, greenhouse gas emissions, and the use of hazardous solvents. As a result, out of this synthesis the carbon footprint of the Progesterone manufacturing process is reduced by more than 70 percent – and eliminating the use of metal catalysts. This product is manufactured via a proprietary bio-catalytical process based on plant sterols.
According to the U.S. Environmental Protection Agency (EPA), Green chemistry is the design of chemical products and processes that reduce or eliminate the use or generation of hazardous substances.
TIEFENBACHER API + INGREDIENTS has been cooperating with the US-based manufacturer for
decades and is proud to start offering green chemistry API´s to our valuable customers in the pharmaceutical industry in Europe.
Enviero Progesterone is immediately available – and our whole portfolio includes more than 600+ API´s to secure the healthcare industry with high-quality solutions along the whole API value chain.
For more information around our raw materials (Human + Veterinary), please contact TIEFENBACHER API + INGREDIENTS at info-api@tiefenbacher.com or visit us on www.tiefenbacher-api.com

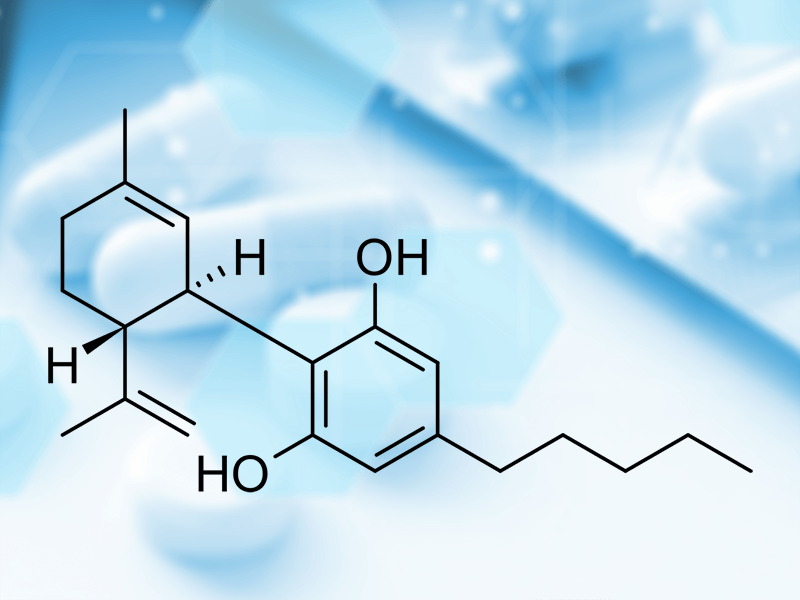
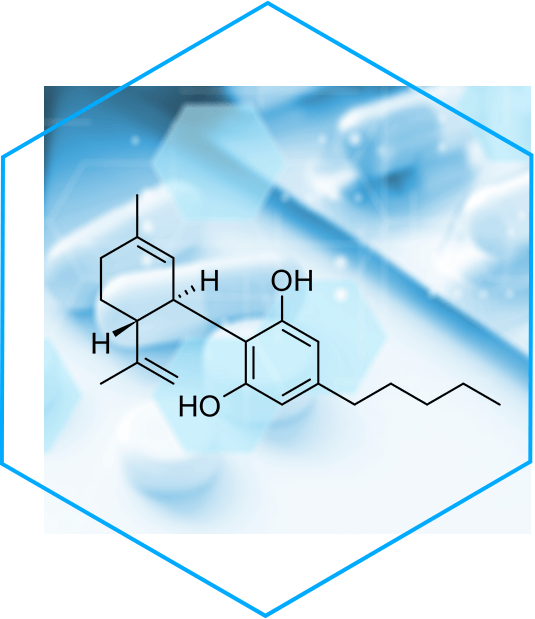
TIEFENBACHER API + INGREDIENTS: GMP certificated Natural Medicinal Cannabidiol (CBD)
TIEFENBACHER API + INGREDIENTS offer GMP certificated Natural Medicinal Cannabidiol (CBD) with highest purity profile in the market
We bring pharma-quality standards of cannabis API for the benefit of Patients in Europe. CBD, short for Cannabidiol (ATC-Code N03AX24), is a chemical compound from the Cannabis sativa plant. It has no psychotropic effects due to it is affinity for cannabinoid receptors CB1 and CB2 and which are part of the endocannabinoid system (ECS).
Pharmacological effect comprehends according to the medical literature positives effects in different autoimmune and neurogenerative pathologies such as multiple sclerosis (spasticity), arthritis, epilepsy (Lennox-Gastaut Syndrome and Dravet Syndrome), schizophrenia and other psychiatric disorders.
TIEFENBACHER API + INGREDIENTS can offer a CBD – white, crystalline substance isolated from industrial hemp in its purest form – manufactured in Europe with a purity > 99,5%, and an impurity profile of THC < 0,01%. By that minimizing side effects for patients.
Our partner for natural CBD is having a MHRA certified EU GMP certificate for both, human and veterinary use and complies with the requirements of the German Medicines Codex (DAC).
TIEFENBACHER API + INGREDIENTS covers the entire supply chain for medical cannabis with the highest pharmaceutical standards: GMP. For more information about our high-quality APIs (Human + Vet) and controlled substances, please contact TIEFENBACHER API + INGREDIENTS: Patricia Wick p.wick@tifi-api.eu
or visit us on: www.tiefenbacher-api.com
TIEFENBACHER API + INGREDIENTS: Dexmedetomidine Hydrochloride – COVID-19 Patients
TIEFENBACHER API + INGREDIENTS secures the supply of critical drug for COVID-19 Patients: Dexmedetomidine Hydrochloride
We ensure hospitals have access to the medicines needed for patient care, including Dexmedetomidine HCL (ATC-Code QN05CM18). TIEFENBACHER API + INGREDIENTS delivers long-term solutions that help deliver top quality patient care throughout the pandemic and beyond.
Dexmedetomidine HCl, a novel sedative-analgesic agent and a front-line drug in our intensive care units becomes increasingly important for patients while it is on the FDA’s Drug Shortage List. The sudden rise is attributable to the global COVID-19 pandemic as Dexmedetomidine HCl injectable is used to help put COVID-19 patients on to medical ventilators.
Its rising tendency is however expected to sustain beyond COVID-19 as tremendous research is being performed based on its advantageous sedating and analgesic properties compared to other alpha 2 agonists.
Dexmedetomidine HCl is currently being investigated and researched in various fields like e.g. Transdermal formulations, Intranasal formulations for treating pain, Oral thin film formulations and Dexmedetomidine slow release microneedle array.
In veterinary practice Dexmedetomidine HCl is used for premedication and as an adjunct to general anesthesia.
Dexmedetomidine Hydrochloride is immediately available – and our whole portfolio includes more than 600+ APIs (Human + Veterinary) to secure the healthcare industry. For more information around our raw materials with value-added service for safe, reliable pharmaceuticals, please contact TIEFENBACHER API + INGREDIENTS: Sandra Thiel s.thiel@tifi-api.eu
or visit us on: www.tiefenbacher-api.com

TIEFENBACHER API + INGREDIENTS: New website – Pioneering API-Distribution since 1963
New Website for TIEFENBACHER PHARMACEUTICALS + INGRIDIENTS: Full-service distributor for high-quality APIs (Human + Vet) + controlled substances with value-added services.
“We are very thrilled to officially announce our new TIEFENBACHER API website, representing the raw material business of TIEFENBACHER GROUP, and aiming to expand our role as a full-service distributor for high-quality Active Pharmaceutical Ingredients (API) and controlled substances with value-added services. We offer more than 600+ APIs (Human + Veterinary) in all fourteen ATC-groups for safe, reliable pharmaceuticals. Our new website communicates a clear message of who we are, what we rise for and where our value lies when delivering on our best value seal: BETTER ACCESS, BETTER QUALITY, and BETTER SUPPLY.
The new API Website is an important milestone within the international brand relaunch of our whole company group (www.tiefenbachergroup.com) and represents the fundamental changes which we have started in the recent years. We are on the move to drive important efficiencies in our supply chain and become a more and more innovative, patient-centered enterprise with a global pharmaceutical footprint.”
Sören Schlosser and Oliver Schrader, Managing Directors TIEFENBACHER API
For more information around our raw materials with best value seal (Better Access – Better Quality – Better Quality), please contact TIEFENBACHER API + INGREDIENTS: info-api@tiefenbacher.com or visit us on: www.tiefenbacher-api.com

Tiefenbacher Group and OnDosis enter a strategic partnership for medicines and digital health
Tiefenbacher Group and OnDosis enter a strategic partnership for game-changing integration of medicines and digital health
Tiefenbacher Group and OnDosis today announced the signing of an extensive co-development agreement for multiple products in the upcoming years. This collaboration strengthens both companies’ commitment to further develop and commercialize innovative products within individualized treatments and digital health, combining traditional drug-based medicines with digital therapeutics and intelligent dosing. The agreement covers future potential collaboration of products within five disease areas, where individualized dosing, improved patient engagement and patient safety are important to improve outcomes. The first co-development project is already initiated within ADHD and targeting the US market. Through the collaboration, OnDosis will benefit from a partner with leading competence in pharmaceutical development, supply chain and B2B commercialization, whilst Tiefenbacher Group gets access to an innovative product concept with game-changing potential in the new era of individualization of treatments and digitalization in healthcare.
“When we met the team from OnDosis the first time, we directly felt a great fi t and the enormous potential in a partnership between both companies. Together with OnDosis we will now establish the first digital platform for individualized medicine and make existing therapies better.”, says Kristian Ruepp, Managing Director at Tiefenbacher Group
“We are very excited by this great opportunity. The collaboration between Tiefenbacher Group and OnDosis builds on a very strong logic, where the joint capabilities of our companies and commitment of our teams will enable acceleration towards of our mission to bring truly game changing therapies to patients”, says Martin Olovsson, CEO at OnDosis.
About Tiefenbacher Group
100% family owned since 1963 Tiefenbacher Group is a global health care company providing innovative and best-in-class solutions along the entire pharmaceutical value chain. That includes the distribution of API´s and the development, manufacturing, and registration of finished dosage forms (FDF). The world´s most trusted health care brands count on Tiefenbacher’s pioneer spirit as well as its pharmaceutical excellence. Leveraging its global presence including own laboratories and manufacturing sites Tiefenbacher is driven to make pharmaceuticals more affordable, more available and better than before. There is one purpose driving Tiefenbacher’s about 600 employee’s day by day: improving the life of millions of patients worldwide.
For further information about TIEFENBACHER GROUP and our solutions for better healthcare and our in-house capabilities to develop and commercialize pharmaceutical products for global markets and e-health solutions, please contact us by email at info@tiefenbacher.com or visit us on: www.tiefenbachergroup.com
About OnDosis
Founded as a spin-out from AstraZeneca in 2017, OnDosis is a Swedish start-up addressing one of the major opportunities in healthcare; personalized dosage of medicines. The proprietary technology platform is centered around a connected handheld dosing device that delivers tailored doses of oral medicines. By combining individual dosing of traditional drug-based medicines augmented by digital therapeutics, OnDosis opens a new chapter in personalized medicine where the mission is to improve patients’ life through delivering the perfect dosage; individualized, intuitive, and intelligent.
Tiefenbacher Group and OnDosis today announced the signing of an extensive co-development agreement for multiple products in the upcoming years. This collaboration strengthens both companies’ commitment to further develop and commercialize innovative products within individualized treatments and digital health, combining traditional drug-based medicines with digital therapeutics and intelligent dosing.
[pdf id=1382]